Guardant wins FDA AdCom backing for colon cancer test

The AdCom voted 8-1 and 6-3 in favour of the Shield test’s safety and effectiveness, respectively. Image Credit: Tada Images / Shutterstock. Despite concerns over false negative results, the Medical Devices Advisory Committee (AdCom) for the US Food and Drug Administration (FDA) has voted in favour of approving Guardant Health’s Shield blood test as a […]
Oncologists support Belong.Life’s AI cancer mentor Dave

Belong.Life’s Dave has received validation from eight senior oncologists for its responses to patients. Image credit: Shutterstock / Andrey Suslov. Belong.Life has received validation from practising oncologists for the company’s conversational AI oncology mentor Dave. The New York-based start-up published an abstract on 23 May for its presentation at the upcoming American Society of Clinical […]
Medline unveils TurboMist small volume nebuliser
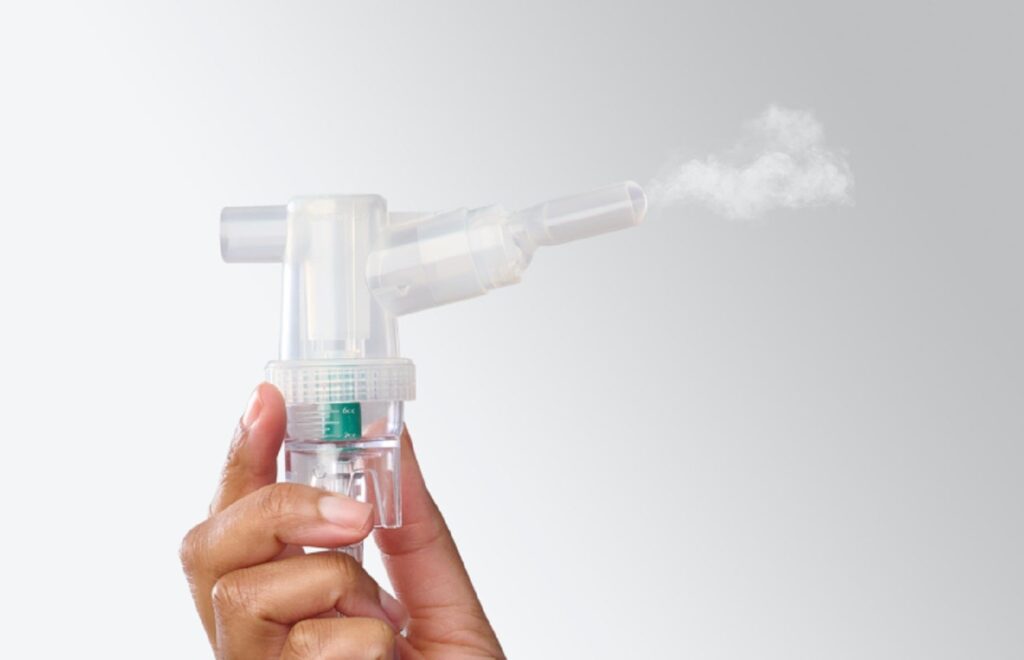
The TurboMist nebuliser is suitable for patients aged five and older. Credit: Medline Industries. Medline has expanded its respiratory care offerings with the introduction of the new Hudson RCI TurboMist small-volume nebuliser. The new device is designed to deliver medication treatments rapidly, with the capability to complete the process in as little as three minutes. […]
FDA approves CorTec’s implant for stroke rehabilitation study
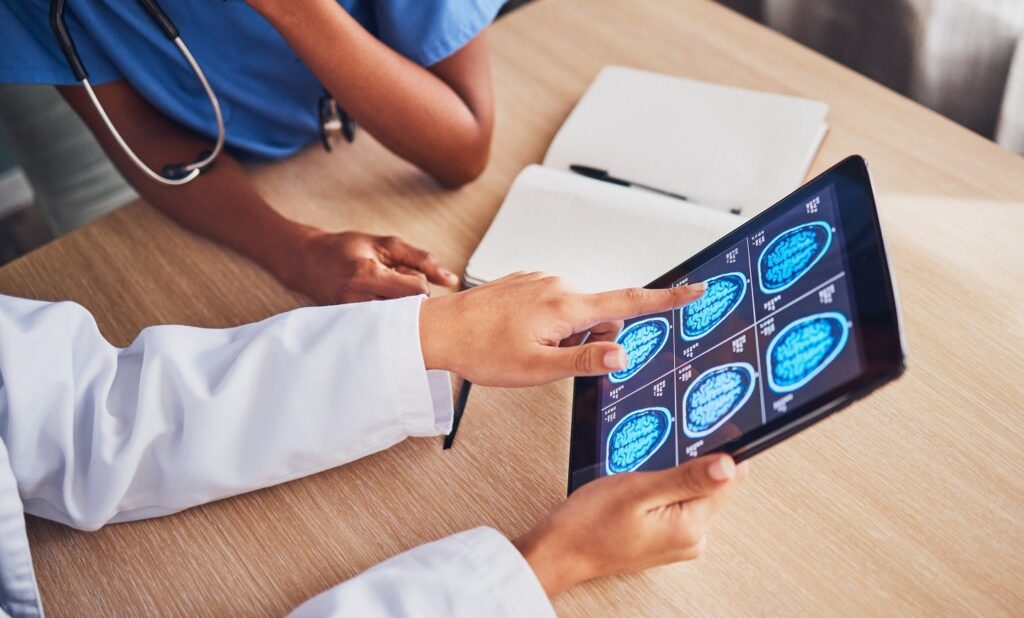
The Brain Interchange implant system will deliver direct cortical electrical stimulation as part of the treatment. Credit: PeopleImages.com – Yuri A/Shutterstock. The US Food and Drug Administration (FDA) has granted approval for an investigational device exemption (IDE) application by the University of Washington School of Medicine to conduct an early feasibility study using CorTec’s Brain […]
Allez Health raises $60m for diabetes sensor technology

Osang Healthcare debuted on the Kosdaq market In March 2024. Image credit: Shutterstock/Olga S L. Biosensor company Allez Health has raised $60m in Series A+ financing to advance its continuous glucose monitoring (CGM) technology. The US-based company, previously known as Zense-Life, says it aims to disrupt cost barriers to CGMs while enhancing performance and the […]
World Scleroderma Day: raising awareness
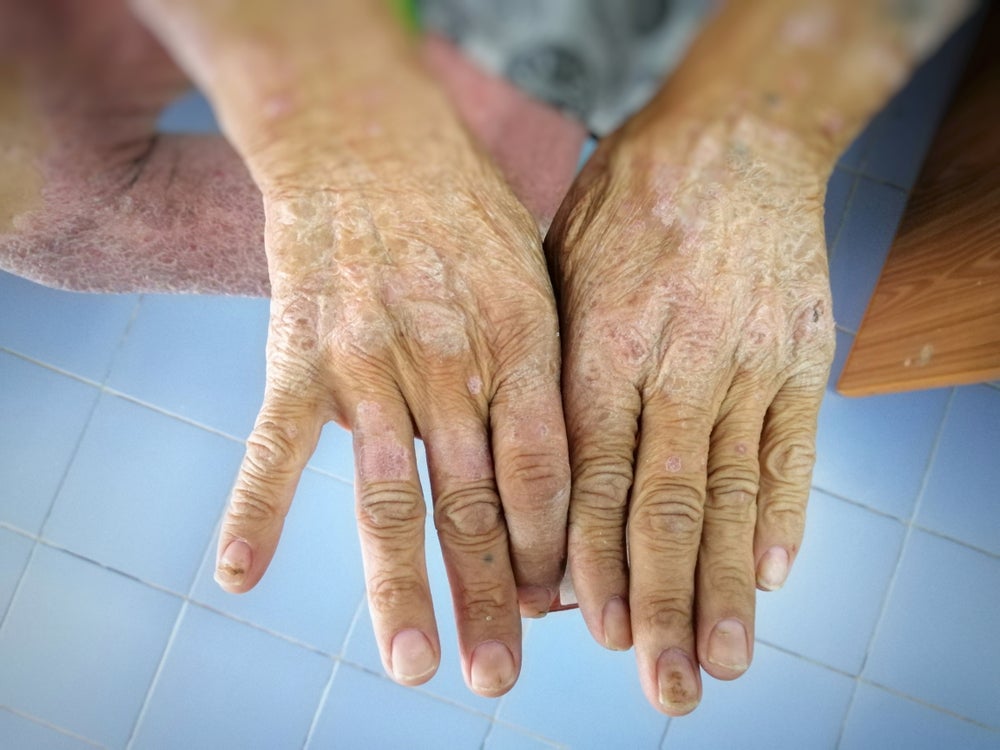
World Scleroderma Day occurs every year on 29 June. Its goal is to raise awareness about the disease, its symptoms and treatments. Scleroderma is a rare, progressive and chronic autoimmune connective tissue disorder that causes excess collagen accumulation. Its course can vary from individual to individual, ranging from minor nuisance symptoms to severe multifaceted disease. […]
FDA classifies Hologic’s recall of BioZorb Marker as Class I

The recall by Hologic is a correction rather than a product removal. Credit: Tada Images/Shutterstock.com. The US Food and Drug Administration (FDA) has classified the recent Hologic BioZorb Marker recall as a Class I event, indicating a high risk of serious injury or death. BioZorb Marker’s manufacturer Hologic has initiated the recall due to complications […]
Click Therapeutics acquires assets from Better Therapeutics
The acquisition includes a prescription digital therapeutic for type 2 diabetes. Credit: Andrey_Popov/Shutterstock.com. Click Therapeutics, a medical technology company, has announced the acquisition of assets from Better Therapeutics, aiming to enhance its development initiatives in cardiometabolic disease and obesity. The acquisition includes a suite of digital therapeutic products, including AspyreRx (BT-001), the first Food and […]
IR-MED’s PressureSafe device cuts pressure injury incidence by half

IR-MED’s PressureSafe is currently undergoing usability studies and is not yet available for commercial use. Image Credit: Robert Kneschke / Shutterstock. Israel-based IR-MED has announced positive results from the usability study of its pressure injury detection device, PressureSafe. PressureSafe uses AI to analyse the infrared spectrographic data collected by the device to detect the presence […]
Invizius completes Phase I trial of dialysis priming product H-Guard
According to a GlobalData market model, the UK dialysis accessories market will be worth $242m in 2033. Credit: Tattoboo via Shutterstock. Scotland-based biotech Invizius has completed a Phase I clinical study, assessing its dialysis priming product H-Guard. H-Guard is a specialised medical device coating indicated for use in dialysis procedures. It is designed to […]
