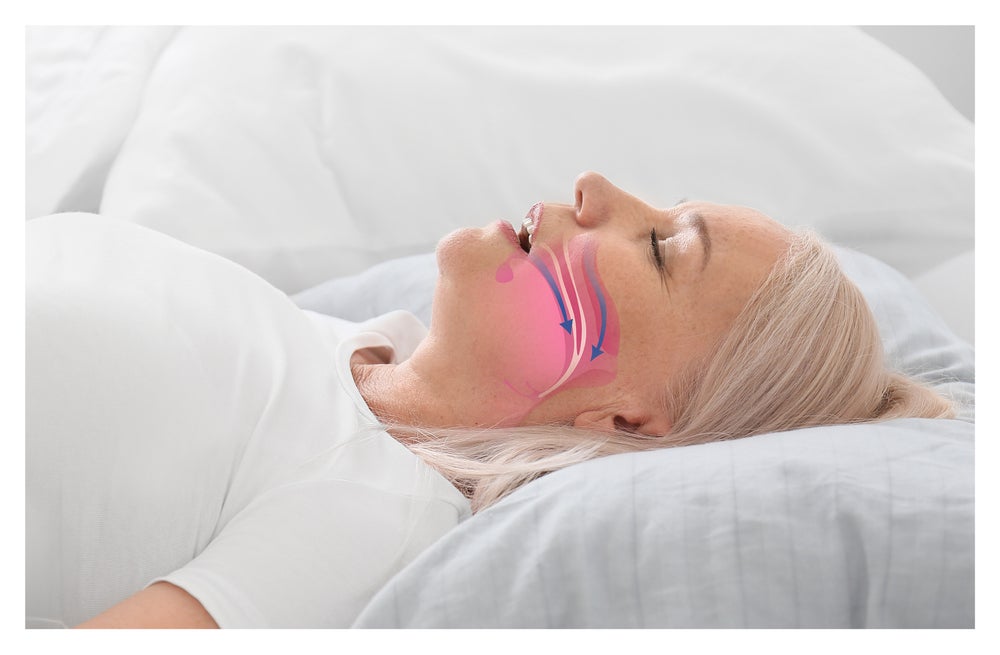
Effective treatments for obstructive sleep apnea (OSA) are essential not only to alleviate immediate symptoms, such as snoring and daytime fatigue, but also to reduce associated risks including cardiovascular disease, diabetes and cognitive impairment. With more than 900 million individuals globally affected by moderate to severe OSA, there is an urgent need to address these health concerns and a push for innovation and investment in the sector.
Neuromodulation is rapidly transforming the OSA landscape by offering distinct advantages over the current standard of care – continuous positive airway pressure (CPAP) therapy. Neuromodulation directly stimulates muscles to ensure the airway remains open during sleep. These devices effectively manage OSA symptoms while overcoming patient hesitancy, discomfort and the poor adherence associated with CPAP therapy, thereby improving patient outcomes and quality of life.
Frequent recalls of conventional OSA treatment devices from major respiratory players, such as Philips and ResMed, have also shaken consumer confidence, intensified the search for more reliable devices and propelled the acceptance of novel treatments. This has created a window of opportunity for alternative solutions to gain market share.
A paradigm shift from conventional therapies
Within the OSA neuromodulation market, the long-time leader, Inspire Medical Systems, has recently shown remarkable revenue growth from $233 million in 2021 to $624 million in 2023. This financial success signals a paradigm shift away from conventional therapies toward more innovative, patient-centric solutions and illustrates the substantial market potential in this segment. As awareness and clinical validation increase, this segment is poised for further expansion and the potential to capture a larger share of the OSA treatment market.
While Inspire continues to lead the market, new competitors are positioning themselves in this emerging market by developing devices with cutting-edge technology. Innovative devices, such as Nyxoah’s minimally invasive and battery-free Genio System, are making significant advances. The Genio System’s positive outcomes for the first 12 months of the DREAM trial (NCT03868618) will help pave the way for its forthcoming US marketing authorisation. Additionally, companies such as StimAire and Vivos Therapeutics are introducing non-surgical alternatives, expanding the treatment options available to patients and clinicians and potentially reshaping the competitive landscape.
This period marks an exciting juncture for neuromodulation markets and OSA treatment, with profound implications for patient care and industry growth. Capitalising on emerging opportunities will require a keen understanding of market trends, patient needs and the competitive landscape. Those who can navigate this dynamic environment effectively will likely emerge as leaders in the evolving market for OSA treatments.
Access the most comprehensive Company Profiles
on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Company Profile – free
sample
Your download email will arrive shortly
We are confident about the
unique
quality of our Company Profiles. However, we want you to make the most
beneficial
decision for your business, so we offer a free sample that you can download by
submitting the below form
By GlobalData

