Tempus launches new HER2 & FOLR1 testing solutions

Tempus’ FOLR1 testing is recommended for patients with fallopian tube, epithelial ovarian, or primary peritoneal cancer. Credit: New Africa / Shutterstock. Technology company Tempus has introduced new testing solutions to help identify patients with HER2 or FOLR1-expressing tumours. The new HER2 and FOLR1 testing solutions will help identify patients eligible for targeted therapies such as […]
RNT Health Insights’ gastric cancer tool gets breakthrough device designation
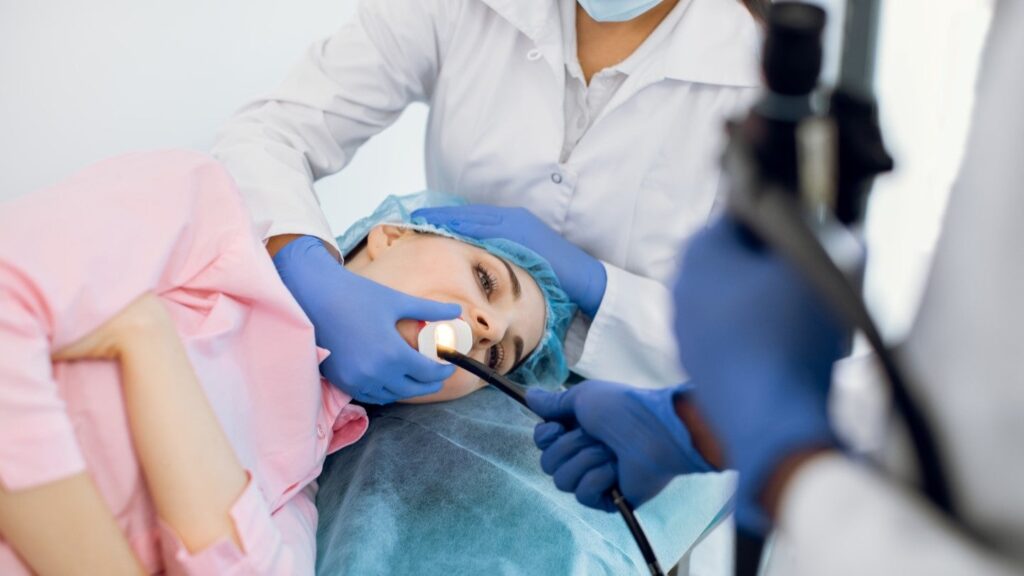
RNT Health Insights’ early gastric cancer detection tool has a 96% accuracy rate. Credit: SofikoS / Shutterstock. India-based clinical-stage medical diagnostics company RNT Health Insights has received breakthrough device designation (BDD) from the US Food and Drug Administration (FDA) for its early gastric cancer detection tool. During routine endoscopy procedures, this technology is designed to […]
Disruption or integration – consumer electronics as medical devices

The successful approval of atrial fibrillation detection on the Apple Watch by the US Food and Drug Administration in 2020 marked a notable milestone for a device aimed at the general public. It was seen as a potential challenge to traditional medical device manufacturers and a glimpse into the future. Today, it stands as an […]
Transseptal access has opened the left side of the heart to transcatheter techniques
Transcatheter surgical techniques refer to minimally invasive procedures performed using catheters and other specialised equipment inserted through blood vessels. Advances in this approach make it an attractive option for patients and practitioners as the technique has fewer associated risks and allows for faster recovery times relative to conventional surgeries. T Transseptal access techniques play a […]
FDA tags OptumHealth’s infusion system recall as Class I

OptumHealth reported no additional injuries or deaths in its recall. Credit: Tada Images / Shutterstock. The US Food and Drug Administration (FDA) has classified OptumHealth’s recall of infusion systems as Class I, the most severe designation available. A total of 208 devices distributed in the US between August 2020 and April 2024 have been recalled. […]
Caristo touts study showing accuracy of its CaRi-Heart technology
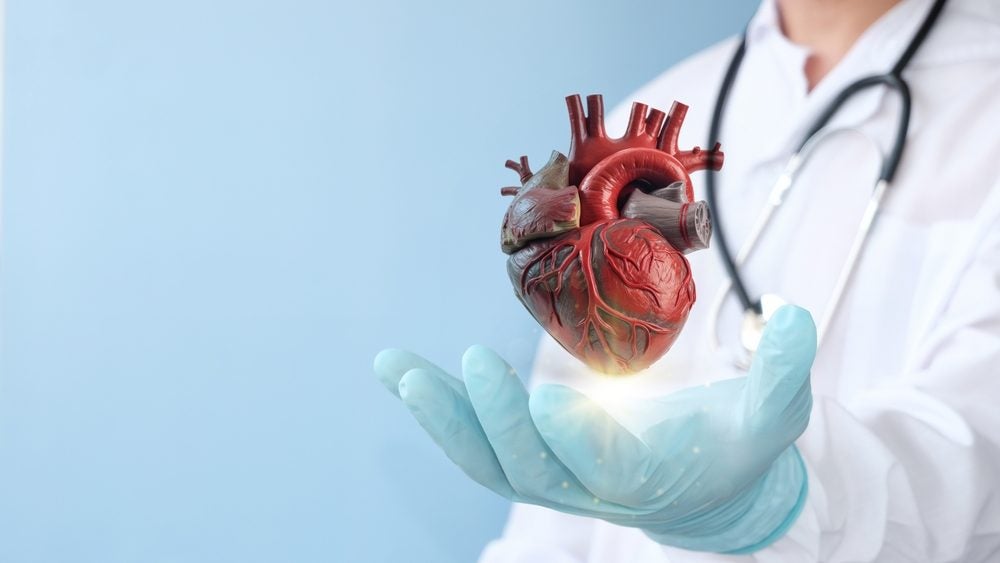
According to GlobalData, there will be 51.8 million cases of heart failure globally by 2027. Credit: Ws Studio1985 via Shutterstock. Caristo has announced that a study demonstrating that its AI CaRi-Heart technology can predict cardiac events due to coronary inflammation at least ten years in advance has been published. Caristo’s CaRi-Heart technology is an AI-assisted […]
MarinHealth Medical Center launches Cardiac MRI tool

The Haynes Cardiovascular Institute at MarinHealth now offers the cardiac MRI to provide cardiac imaging services. Credit: Michal Jarmoluk / Pixabay. MarinHealth Medical Center has announced the expansion of its cardiac care services with the introduction of its cardiac magnetic resonance imaging (MRI) tool. The new noninvasive diagnostic tool is designed to offer heart’s high-resolution […]
Avita obtains FDA approval for cell harvesting device Recell GO System
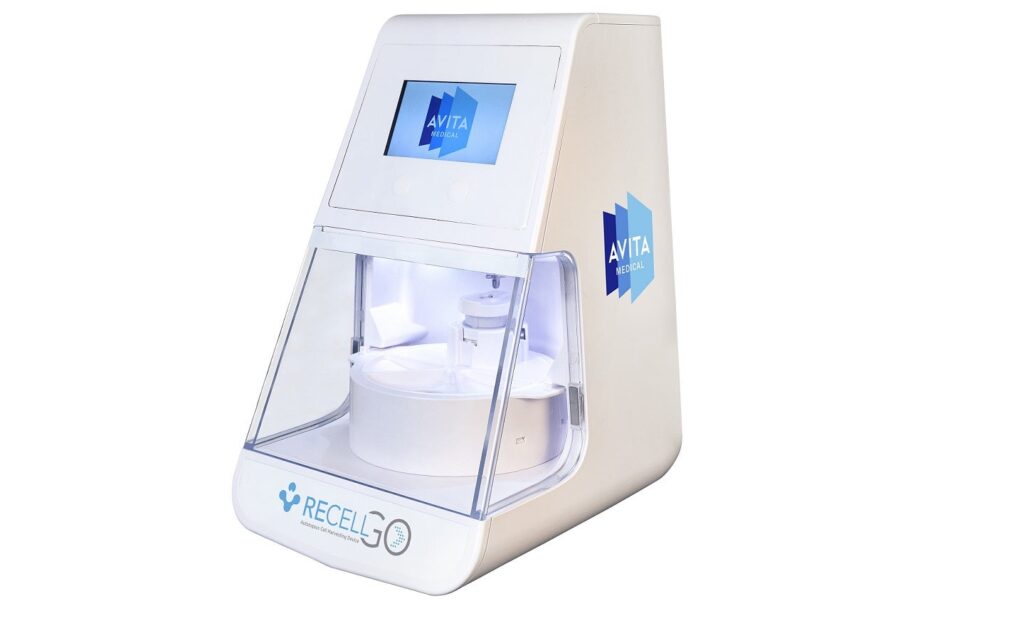
The Recell GO System can treat skin wounds and defects by harnessing the regenerative properties of a patient’s own skin. Credit: GlobeNewswire / Avita Medical, Inc. Avita Medical has secured approval from the US Food and Drug Administration (FDA) for a premarket approval (PMA) supplement for its next-generation autologous cell harvesting device, the Recell GO […]
Allurion expands AI virtual care to GLP-1RA patients

Research conducted by the US insurance provider group, Blue Cross Blue Shield, found that 58% of patients discontinue the use of GLP-1RAs before reaching a clinically meaningful health benefit. Credit: Shutterstock / Motortion Films Massachusetts-based gastric device firm, Allurion, has announced that its artificial intelligence (AI) driven personal coach, named Iris, is being offered to […]
Sherlock starts clinical trial for rapid OTC diagnostics for STIs

Sherlock’s rapid OTC test detects the presence of chlamydia trachomatis and neisseria gonorrhoeae, as well as provides results in under 30 minutes. Image Credit: Jarun Ontakrai / Shutterstock. Sherlock Biosciences has enrolled its first patient in the PROMISE trial which evaluates the company’s rapid over-the-counter (OTC) test for sexually transmitted infections (STIs). The molecular test […]
