Medtech Europe CEO urges for industry engagement ahead of EU elections

The MedTech manifesto calls on European politicians and policymakers to put medical device advances at the forefront of healthcare policy agendas. Credits: GlobalData. The 2024 MedTech Forum has kicked off in Vienna, Austria, with MedTech Europe CEO Oliver Bisazza urging visitors to keep a keen eye on the upcoming European Union (EU) elections, as the […]
Indica Labs’ HALO AP Dx platform receives FDA 510(k) clearance
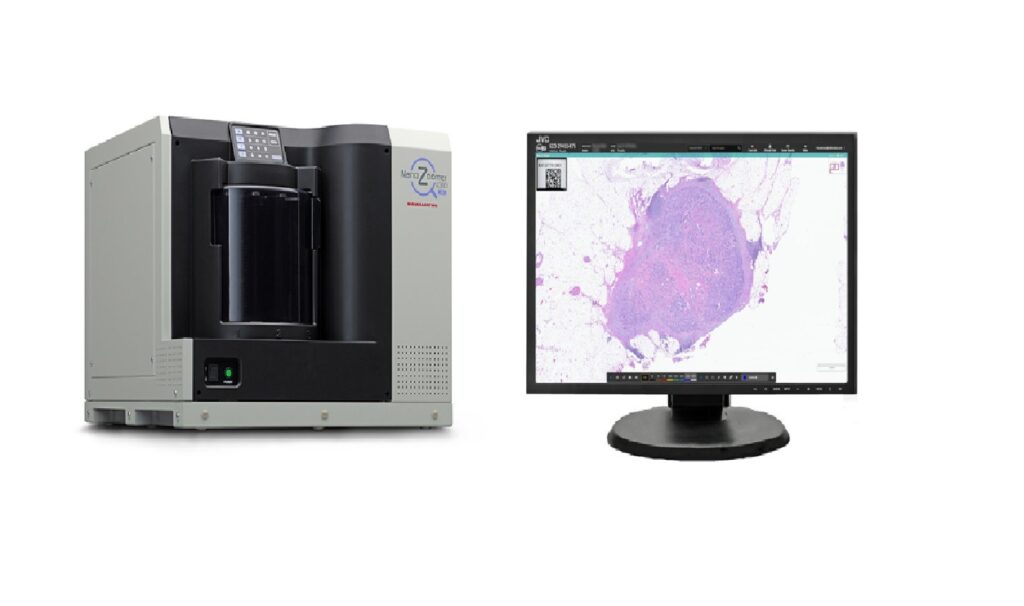
The HALO AP Dx software is designed for review of images acquired with the NanoZoomer S360MD Slide scanner. Credit: Indica Labs. Indica Labs has received 510(k) clearance from the US Food and Drug Administration (FDA) for its enterprise digital pathology platform, HALO AP Dx. This approval allows the platform to be used for primary diagnosis […]
KT unveils two new patches for diabetes care

The patches will revolutionise how individuals with diabetes manage their condition. Credit: Tesa Robbins from Pixabay. Kinesiology tape brand KT has introduced two new patches designed to enhance diabetes care, expanding its KT Health Line products. The company said that the continuous glucose monitor (CGM) and insulin pump patches will revolutionise how individuals with diabetes […]
Medical Device Network Excellence Awards 2024: HemoSonics

HemoSonics, a medical device technology company based in the United States, has won an award in the Innovation category in the 2024 Medical Device Network Excellence Awards for its Quantra Hemostasis System. This diagnostic device is the only one of its kind cleared by the U.S. Food and Drug Administration for use across four major […]
Onward’s ARC-EX therapy hits success in pivotal trial for spinal cord injury

Onward’s ARC-EX delivers targeted, programmed spinal cord stimulation non-invasively. Image credit: Shutterstock/Sebastian Kaulitzki. Onward Medical’s ARC-EX therapy is going from strength to strength after results from a pivotal trial investigating the device demonstrated significant improvements in recovery from spinal injuries. The US-based company released a snapshot of the Up-LIFT trial’s data in January 2024, but […]
The impact of congenital cytomegalovirus

Cytomegalovirus (CMV) is a common virus that typically causes mild or asymptomatic infections in people with healthy immune systems. However, congenital CMV can have serious consequences for newborns such as permanent hearing loss, neurodevelopmental delays, and cerebral palsy. Congenital CMV occurs when infected mothers transmit the virus to their unborn child, and it is the […]
GE HealthCare and Medis link for non-invasive coronary testing

GE HealthCare, a leader in medical technology, and Medis, a specialist in medical imaging software, have announced a partnership to improve non-invasive coronary assessments. This collaboration represents a major advancement in cardiovascular diagnostics, showcasing the potential of integrating imaging technologies with advanced software solutions. The advanced imaging systems from GE HealthCare, along with Medis’s pioneering […]
Roche’s Lp(a) test wins FDA breakthrough device designation
The test will measure the number of Lp(a) molecules per litre of blood. Credit: angellodeco/Shutterstock.com. Roche has received the US Food and Drug Administration’s (FDA) breakthrough device designation for its Tina-quant lipoprotein Lp(a) RxDx assay, a diagnostic tool for cardiovascular disease risk. This designation is a significant step towards the approval of the test, which […]
Cochlear concludes Oticon Medical cochlear implant acquisition

The transaction, which involved no headline purchase price, marks the end of Demant’s involvement in hearing implants. Credit: adriaticfoto / Shutterstock.com. Hearing solutions provider Cochlear has concluded the acquisition of the Oticon Medical cochlear implant business from Demant. The transaction, which involved no headline purchase price, marks the end of Demant’s involvement in the hearing […]
BSI launches new regulatory standard team for software and AI

Speaking with Medical Device Network, operations delivery director of regulatory services, Haydar Jaafar, detailed how SaMD has taken top priority for the group. Credit: Shutterstock / fizkes The British Standards Institute (BSI) has established a specialised regulatory team aimed at regulating software used as a medical device (SaMD) alongside artificial intelligence (AI) in a bid […]
